CASE SERIES
Extra Nasal Rhinosporidiosis; A Clinical Challenge How We Managed
Dr Nidhin SB1, Dr Ankur Chandrakar2
Abstract
Rhinosporidiosis is a chronic granulomatous disease caused by Rhinosporidium seeberi, endemic in the the states of Chhattisgarh, Madhya Pradesh, West Bengal,, and Tamil Nadu1. The most common presentation is a nasal mass with epistaxis2, but extra-nasal rhinosporidiosis often poses a clinical challenge, mimicking various other conditions3. This study presents three cases of extra-nasal rhinosporidiosis involving the nasolacrimal duct, pharyngeal, and laryngotracheal regions, each with unique clinical presentations and management approaches. The treatment of choice is complete excision with wide cauterization of the base to minimize recurrence. Predictive factors for extra-nasal rhinosporidiosis include middle age, male gender, multifocal lesions, and a history of recurrent surgery4. Comprehensive management involving multiple specialties is crucial for successful treatment and prevention of recurrence.
Introduction
Rhinosporidiosis is a chronic unusual granulomatous infection brought about by Rhinosporidium seeberi, a microorganism first documented by Guillermo Seeberi in Argentina back in 19905. Despite bearing morphological similarities to fungi and protozoa, it falls under its distinct classification aquatic protozoan parasite in the mesomycetozoea class6. Interestingly, records of this ailment date back over a century, long before the identification of its causative agent in 1990. Rhinosporidiosis predominantly afflicts both humans and domestic animals residing in regions endemic to India and Sri Lanka. In India endemic in states of Chhattisgarh, Madhyapradesh, West Bengal and Tamilnadu4.
This disease typically surfaces on the mucous membrane, with the nose and nasopharynx serving as its most common haunts. However, isolated instances have been reported in various anatomical locations, spanning the eye, ear, trachea, bronchi, genitourinary tract, larynx, and parotid gland. Clinically, it manifests as a soft, polypoid mass, with the nasal region being the most frequently affected site6. It’s essential to underscore that traditional antifungal and antimicrobial therapies prove futile against R. seeberi. Consequently, the sole efficacious treatment for rhinosporidiosis entails surgical excision of the impacted area, complemented by thorough cauterization to forestall any recurrence7. Here we discuss 3 different presentations of extra nasal rhinosporidiosis.
Case Series:
Case 1:
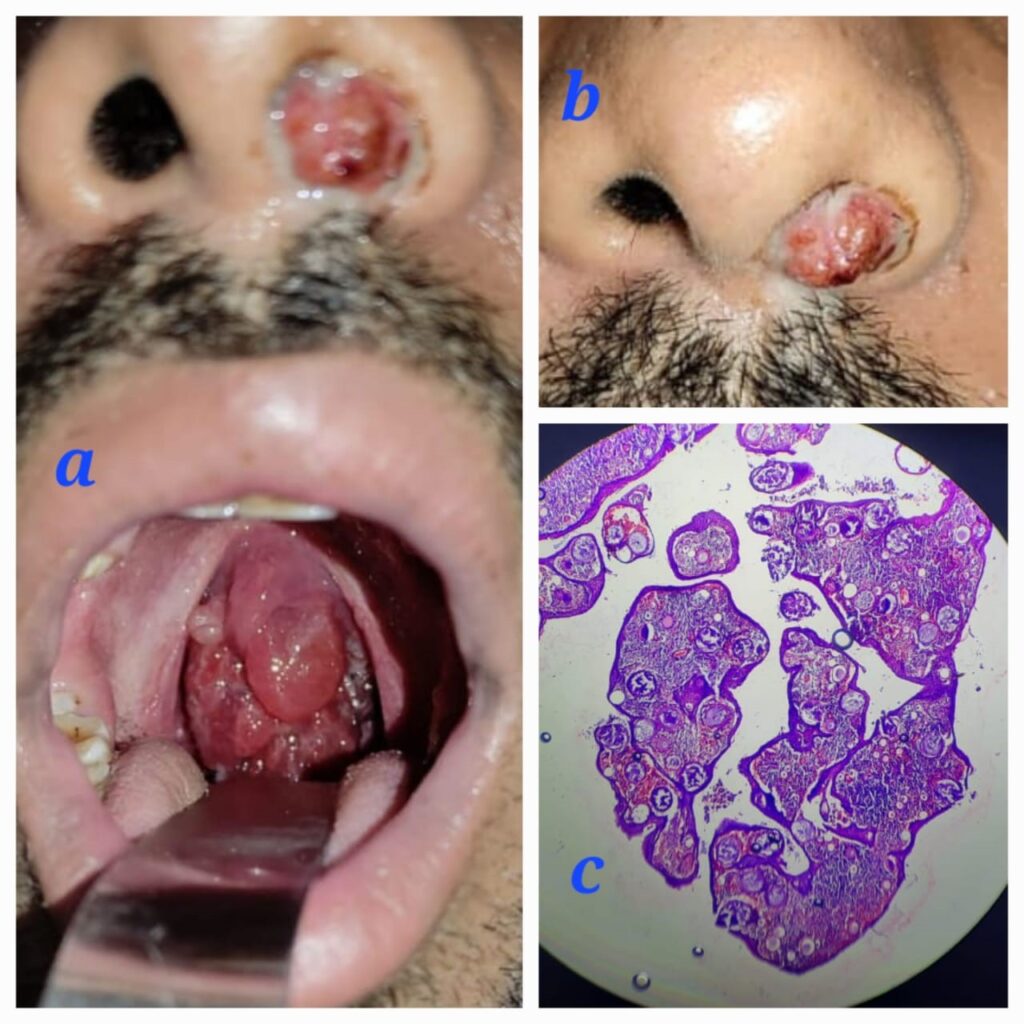
A 24-year-old male patient presented with complaints of left-sided nasal obstruction, which progressed to difficulty in swallowing, associated voice changes, snoring, and sleep apnea. The patient also provided a history of nasal surgery performed 2 years prior, although no documents were available. Upon examination, a fleshy nasal mass was found protruding from the left nasal cavity, which bled upon probing. Intraorally, the mass completely hung from the nasopharynx, filling the oropharynx and having an attachment to the posterior pillar of the tonsil Fig 1a,b.
A contrast-enhanced CT scan of the Nose PNS and Neck revealed attachments to the inferior turbinate, middle turbinate, torus tubaris of the left nasal cavity, posterior pharyngeal wall, and the left side of the posterior tonsillar pillar. The patient underwent a combined endoscopic trans-nasal and trans-oral excision of the mass. Intraoperatively, the attachment of the mass was noted to originate from the inferior meatus in the nasal cavity and from the torus tubaris in the nasopharynx. Initially, the nasal cavity mass was excised after cauterizing the pedicle. During the procedure, a partial inferior turbinectomy was also performed to ensure complete visualization of the inferior meatus and to prevent residual disease. The trans-oral route was approached by placing a Boyle Davis mouth gag, and an infant feeding tube was passed through the nasal cavity to retract the uvula and soft palate. Using a 70-degree endoscope, the oropharyngeal and nasopharyngeal mass was excised, and the base was cauterized with bipolar cautery. Histopathological examination (HPE) showed sporangia with its characteristic bilamellar thick chitinous wall, Fig 1c and the sporangiospores were visualized using special stains such as Gomori methenamine silver. The patient was followed up for 6 months, and no recurrence has been observed.
Case 2
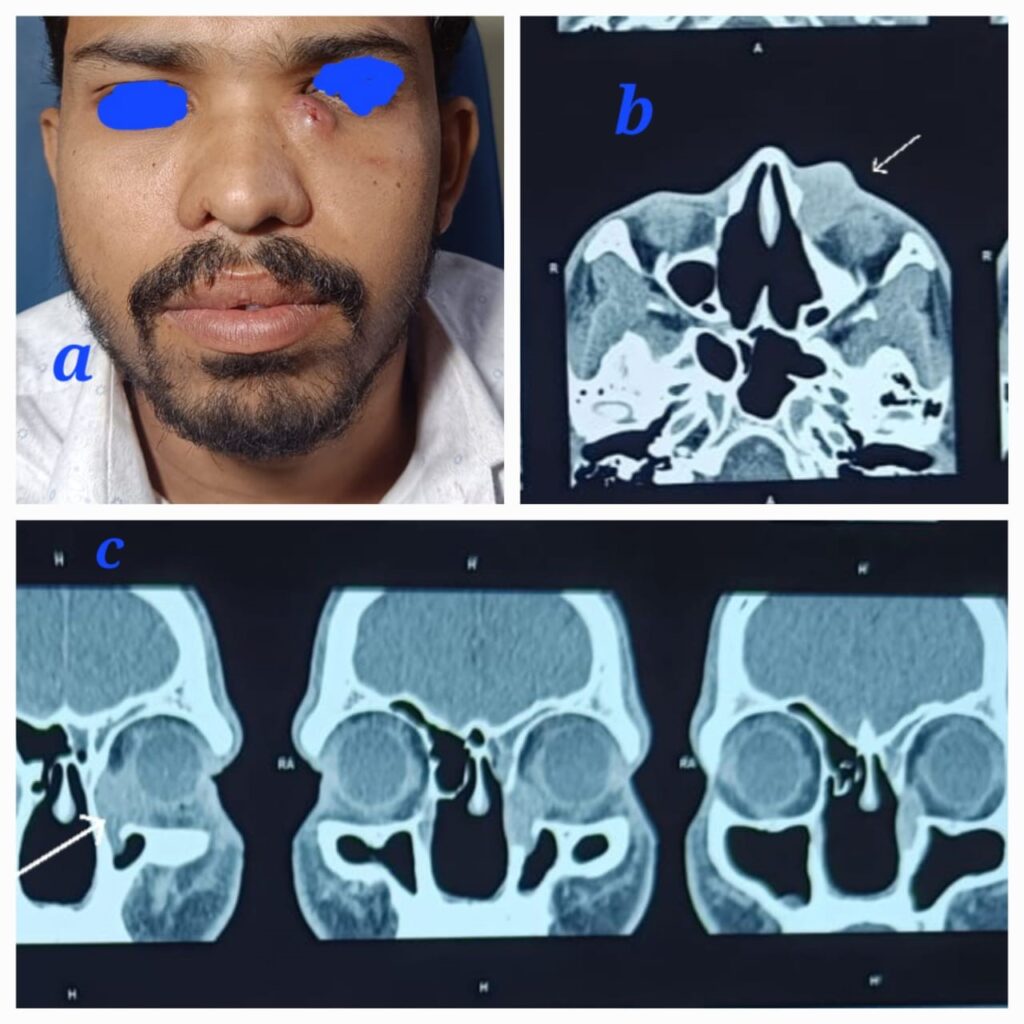
A 30-year-old male patient, a known case of recurrent rhinosporidiosis who had undergone three previous surgeries in our hospital, presented with a unique complaint. He had been lost to follow-up after his last endoscopic excision of nasal rhinosporidiosis eight months prior. The patient presented with complaints of an external nasal swelling below the left eye. Upon examination, a tender, soft cystic ovoid swelling measuring 2×3 cm was observed in the left medial canthus region Fig 2a. Diagnostic nasal endoscopy did not reveal any nasal mass. A contrast-enhanced CT scan of the Nose PNS and Orbit showed an ovoid hyperdense soft tissue lesion measuring 25x22x9 mm in the left nasolacrimal duct and medial canthus, with post-contrast enhancement noted Fig 2b,c.
The patient underwent a combined endoscopic dacrocystectomy and external mass excision through a medial canthal incision, resulting in the complete removal of the mass. Postoperatively, significant infraorbital edema was noted. The patient was followed up for 6 months, and no recurrence was observed.
Case 3
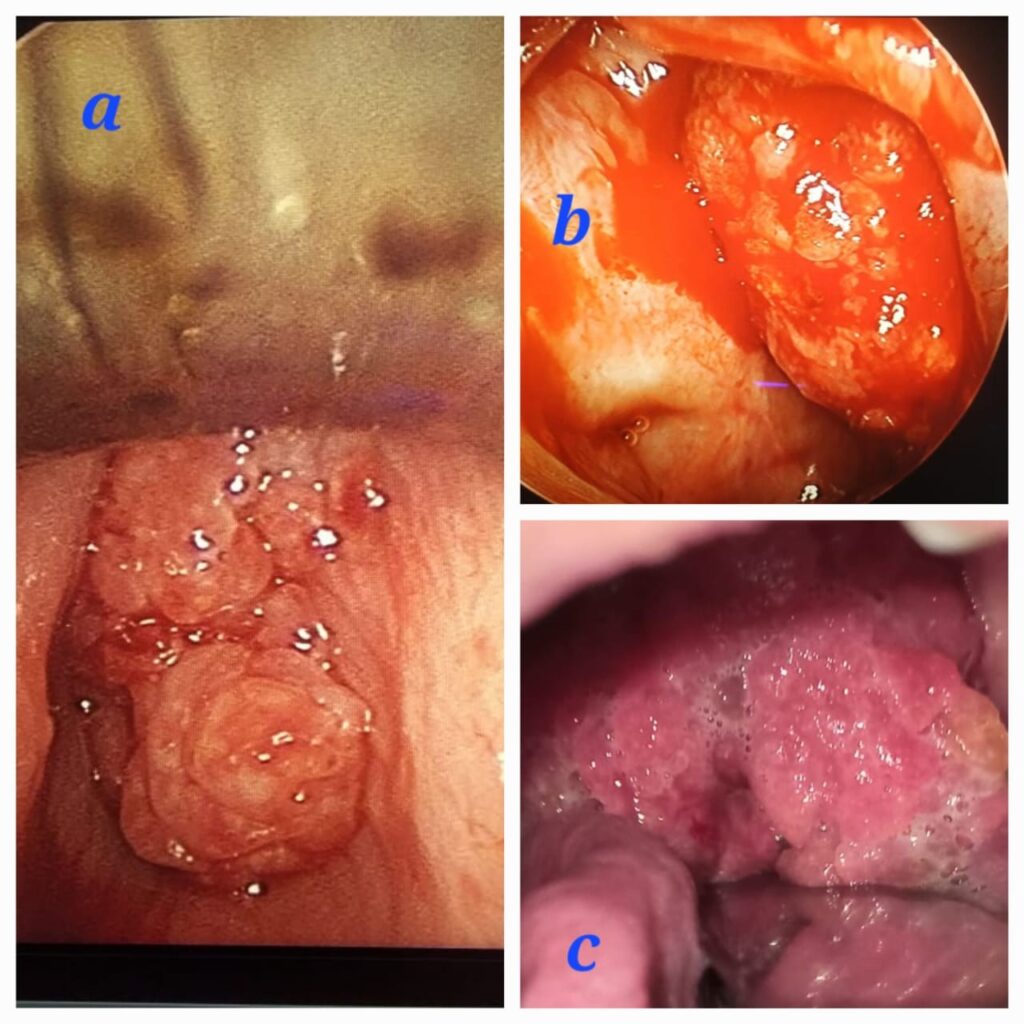
A 42-year-old male patient, a known case of recurrent rhinosporidiosis who had undergone six previous surgeries, presented to the casualty department with stridor. Emergency tracheostomy was performed the following day. Upon examination, a red fleshy granular mass was observed in the left nasal cavity, along with an oropharyngeal mass arising from the nasopharynx Fig 3c. Further video laryngoscopy revealed a laryngeal papillomatous lesion arising from the subglottic region. Radiological evaluation, including a CECT of the Nose PNS and Neck, showed a hyperdense lesion filling the left nasal cavity, nasopharynx, and subglottic region. The patient underwent a combined endoscopic trans-nasal and trans-oral procedure, followed by direct laryngoscopy to debulk the laryngeal rhinosporidiosis using cold MLS instrumentation Fig 3a,b.
Three months post-surgery, airway evaluation was performed, and the patient’s tracheostomy was successfully decannulated. The patient is currently on regular follow-up.
Discussion
Rhinosporidiosis is a chronic and localized granulomatous disease affecting the mucocutaneous region. It is commonly caused by Rhinosporidium seeberi, initially thought to be a water mold but later suggested to be an aquatic protistan parasite in the mesomycetozoea class8. This disease is endemic in Chhattisgarh, Madhya Pradesh, West Bengal, Tamil Nadu, and Odisha6.
The transmission of infection occurs primarily through three routes:
a) Direct contact: Demellow’s theory postulates that when the mucosa comes in contact with the organism or its spore, direct transmission occurs through minor epithelial breaches, leading to nasal and nasopharyngeal rhinosporidiosis.
b) Autoinoculation: This is responsible for satellite lesions around the nasal cavity, skip lesions around the nasopharynx, oropharynx, hypopharynx, laryngotracheal region, and ocular lesions.
c) Hematogenous spread: This is responsible for disseminated rhinosporidiosis and cutaneous rhinosporidiosis4.
Rhinosporidiosis is commonly observed in young and middle-aged male patients from rural areas. This is often associated with the practice of communal bathing in village ponds, where animals and humans bathe together. Spores from animal feces and urine mix with pond water, and when they come into contact with traumatized nasal mucosa, they enter the body6.
Clinically, rhinosporidiosis is divided into Nasal and Extra Nasal types, which include Pharyngeal, Laryngo-Tracheal, Lacrimal duct, Parotid duct, and Disseminated forms. Factors predicting Extra Nasal Rhinosporidiosis include middle age (>30 years) population, male sex, symptoms lasting more than 10 months, and multifocal origin of the mass4. The most common site for extra nasal rhinosporidiosis is the nasopharyngeal region, followed by the nasolacrimal duct, parotid duct, and musculoskeletal system. Since rhinosporidiosis is a slow-growing lesion, it typically presents insidiously with epistaxis being the most common symptom, followed by nasal obstruction. However, in extra nasal rhinosporidiosis, symptoms may vary depending on the site involved. This presents a clinical dilemma and challenge to treating surgeons as it can mimic different conditions. Therefore, it is essential to maintain a high level of suspicion when dealing with patients from endemic regions or those with a history of pond bathing or previous multiple nasal surgeries.
The management of extra nasal rhinosporidiosis requires a holistic approach7. It involves treating the primary site lesion along with any spread lesions, necessitating complete excision to prevent recurrence. In cases of musculoskeletal and disseminated rhinosporidiosis, a multidisciplinary approach is essential, involving general surgeons and orthopedic surgeons to perform the necessary procedures.
Conclusion
- In cases of recurrent nasal rhinosporidiosis, it is imperative to carefully examine for skip lesions in the nasopharynx, oropharynx, and hypopharynx.
- Complete excision of the mass with wide cauterization of the base remains the treatment of choice.
- Extra Nasal Rhinosporidiosis is frequently observed in patients with recurrent rhinosporidiosis, primarily due to autoinoculation or the spillage of rhinosporidial spores.
- The most common predictive factors for extra nasal rhinosporidiosis include middle-age population, male gender, multifocal origin of the mass, and a history of recurrent surgeries.
Bibliography
- Mathew S, Arora RD, Prabha N, Kamble P, Satpute SS, Nagarkar NM. Retroanalytical Study of Epidemiological Factors of Rhinosporidiosis.Int Arch Otorhinolaryngol.2021;25(04):e504-e508. doi:10.1055/s-0040-1718526
- Saha J, Basu AJ, Sen I, Sinha R, Bhandari AK, Mondal S. Atypical Presentations of Rhinosporidiosis: A Clinical Dilemma? Indian J Otolaryngol Head Neck Surg.2011;63(3):243-246. doi:10.1007/s12070-011-0275-x
- Sinha A, Phukan J, Bandyopadhyay G, et al. Clinicopathological study of rhinosporidiosis with special reference to cytodiagnosis. J Cytol. 2012;29(4):246. doi:10.4103/0970-9371.103943
- Mehta R, Sb N, Nagarkar NM. Clinical Presentation: ENT Spectrum. In: Nagarkar NM, Mehta R, eds. Rhinosporidiosis. Springer Singapore; 2022:33-45. doi:10.1007/978-981-16-8508-8_4
- Putthia H, Manjunatha BS, Astekar M, Taufiq S. Palatal rhinosporidiosis: an unusual case report and review of the literature. J Korean Assoc Oral Maxillofac Surg. 2018;44(6):293. doi:10.5125/jkaoms.2018.44.6.293
- Varghese L, Kurien R, Susheel S, Cherian LM, Rebekah G, Rupa V. Rhinosporidiosis—Factors predicting disease recurrence. Mycoses. 2021;64(12):1471-1479. doi:10.1111/myc.13381
- Arora RD, Nagarkar NM, Chandran M. Treatment of Rhinosporidiosis. In: Nagarkar NM, Mehta R, eds. Rhinosporidiosis. Springer Singapore; 2022:77-81. doi:10.1007/978-981-16-8508-8_9
- Giri AK, Padhan S, Galhotra A. Epidemiology of Rhinosporidiosis. In: Nagarkar NM, Mehta R, eds. Rhinosporidiosis. Springer Singapore; 2022:7-16. doi:10.1007/978-981-16-8508-8_2
Article Info
Published on 01.10.2023
How to cite this article: Nidhin S B, Chandrakar A. Extra Nasal Rhinosporidiosis; A Clinical Challenge How We Managed. JAMST. 2023; 1(1):19-22
Conflict of interest: Nil
Funding : Nil
Ethical consideration: All subject who underwent treatment at our hospital was informed and proper consent was taken before sending the paper for publication.
Author Info
1Dr Nidhin SB
Assistant Professor PtJNM Government Medical College, Raipur Chhattisgarh
2Dr Ankur Chandrakar
Assistant Professor PtJNM Government Medical College Raipur Chhattisgarh, Orcid id: 0000-0001-7721-2712, Email id: ankur.chandrakar006@gmail.com
Corresponding Author: Dr NIDHIN SB, Email id: sbnidhin@gmail.com, Phone No: 9846822147, Orcid id: 0000-0001-8829-6798